- Phosphate[7]
- Inorganic Acids[7]
- Agrochemicals & Pesticides[10]
- Water Treatment[6]
- Polymer[1]
- Other Chemicals[2]
- Contact Person : Ms. Pei Cathy
- Company Name : Shanghai Kima Chemical Co., Ltd.
- Tel : 0086-21-52661975
- Fax : 0086-21-52661949
- Address : Shanghai,Shanghai,RM2219,22F ZhongYi Mansion, No. 1040, Caoyang Road
- Country/Region : China
- Zip : 200063
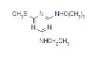
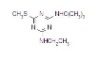
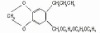
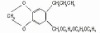
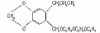
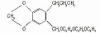
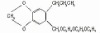
98% Terbutryn
Terbutryn
CAS #: 886-50-0
Categories: Herbicides
Description:
NOMENCLATURECommon name: terbutryn; terbutryneIUPAC name: N2-tert-butyl-N4-ethyl-6-methylthio-1,3,5-triazine-2,4-diamineChemical Abstracts name: N-(1,1-dimethylethyl)-N'-ethyl-6-(methylthio)-1,3,5-triazine-2,4-
diamine
PHYSICAL CHEMISTRYComposition: Tech. is 98%.
Mol. wt.: 241.4;
M.f.: C10H19N5S;
Form: White powder.
M.p.: 104-105C.
B.p.: 274C/101 kPa.
V.p.: 0.225 mPa (25C) (OECD 104).
KOW: logP = 3.65 (25 oC, unionised).
Henry: 1.5x10-3 Pa m3 mol-1 (calc.)
S.g./density: 1.12 (20C).
Solubility: In water 22 mg/l (22C). In acetone 220, hexane 9, n-octanol 130, methanol 220, toluene 45 (all in g/l, 20C). Also readily soluble in dioxane, diethyl ether, xylene, chloroform,
carbon tetrachloride, dimethylformamide. Slightly soluble in petroleumether.Stability:
Stable under normal conditions. The methylthio group is hydrolysed in the presence of
strong acids or alkalis. At 70C, no significant hydrolysisCcurs at pH 5, 7, or 9. pKa:
4.3, weak base.
APPLICATIONSBiochemistry: Photosynthetic electron transport inhibitor at the photosystem II receptor
site.Mode of action: Selective herbicide, absorbed by the roots and foliage, with translocation
acropetally through the xylem, and accumulation in the apical meristems.Uses: Used pre-emergence in winter cereals, at 1-2 kg a.i./ha, to control blackgrass and annual meadow grass. Among the autumn-germinating broad-leaved weeds controlled are chickweed, mayweed, poppies and speedwell, but cleavers are rather resistant. Other pre-emergence uses are on sugar cane and sunflowers; and, in mixture with terbuthylazine, on beans, peas and potatoes. In mixture with metolachlor, used in cotton and peanuts. Also used post-emergence (0.2-0.4 kg/ha) in cereals, (1-3 kg/ha) in sugar cane, and as a directed spray in maize. As 'Clarosan', it is used to control algae and submerged vascular plants in waterways, reservoirs and fish ponds.Phytotoxicity: Not safe for post-emergence use in cereals which are under stress.Formulation types: FW; GR; MG; SC; WP.
MAMMALIAN TOXICOLOGYOral: Acute oral LD50 for rats 2500, mice 500 mg/kg.Skin and eye: Acute percutaneous LD50 for rats >2000, rabbits >20 000 mg/kg. Not a skin or eye irritant (rabbits). Not a skin sensitiser (guinea pigs).Inhalation: LC50 (4 h) for rats >2200 mg/m3 air.NOEL: (2 y) for rats 300 ppm, for mice 3000 ppm; (1 y) for dogs 100 mg/kg diet daily.ADI: 0.027 mg/kg b.w.Other: Non-mutagenic.Toxicity class: WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGYBirds: Dietary LC50 (5 d) for bobwhite quail 5000, mallard ducks >4640 mg/kg b.w.Fish: LC50 (96 h) for rainbow trout 1.1, bluegill sunfish 1.3, carp 1.4.Daphnia: LC50 (48 h) 2.66 mg/l.Algae: EC50 (7 d) for Selenastrum capricornutum 0.013 mg/l.Other aquatic spp.: EC50 (48 h) for Quahog clam 5.6 mg/l.Bees: Not toxic to bees. LD50 (oral) >225 ug/bee; (contact) >100 ug/bee.Worms: LC50 for Eisenia foetida 170 mg/kg.
ENVIRONMENTAL FATEAnimals: In mammals, following oral administration, 73-85% is eliminated as the dealkylated hydroxy metabolite in the faeces within 24 hours.Plants: In plants, terbutryn is degraded in a manner similar to other methylthio-s-
triazines, viz. by oxidation of the methylthio group to hydroxy metabolites, and by
dealkylation of the side-chains. Conjugates are also formed.Soil/Environment: Soil micro-organisms play an important role in the degradation of
terbutryn. Residual activity in soil is 3-10 weeks, depending upon rate of application,
soil type, and weather. DT50 in soil 14-50 d. Koc 2000, indicating a low leaching
potential. Degradation in aquatic systems is caused by microbial processes; photolysis also contributes. Considerable amounts of terbutryn are removed from the water by adsorption to the sediment.
98% Terbutryn