- Phosphate[7]
- Inorganic Acids[7]
- Agrochemicals & Pesticides[10]
- Water Treatment[6]
- Polymer[1]
- Other Chemicals[2]
- Contact Person : Ms. Pei Cathy
- Company Name : Shanghai Kima Chemical Co., Ltd.
- Tel : 0086-21-52661975
- Fax : 0086-21-52661949
- Address : Shanghai,Shanghai,RM2219,22F ZhongYi Mansion, No. 1040, Caoyang Road
- Country/Region : China
- Zip : 200063
Terbuthylazine (Tech.97 % pure) herbicide
Terbuthylazine
CAS #: 5915-41-3
Categories: Herbicides
Description:
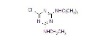
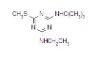
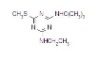
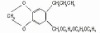
NOMENCLATURECommon name: terbuthylazineIUPAC name: N2-tert-butyl-6-chloro-N4-ethyl-1,3,5-triazine-2,4-diamineChemical Abstracts name: 6-chloro-N-(1,1-dimethylethyl)-N'-ethyl-1,3,5-triazine-2,4-diamine
PHYSICAL CHEMISTRYComposition: Tech. is 97 % pure.
Mol. wt.: 229.7; M.f.: C9H16ClN5
Form: Colourless powder.
M.p.: 177-179C; V.p.: 0.15 mPa (25C)
KOW: logP = 3.21 (unionised); Henry: 4.05x10-3 Pa m3 mol-1 (calc.)
S.g./density: 1.188 (20C).
Solubility: In water 8.5 mg/l (pH 7, 20C). In acetone 41, ethanol 14, n-octanol 12, n-hexane 0.36 (all in g/l, 25C).
Stability: Stable in neutral, weakly acidic and weakly alkaline media; hydrolysed in acidic or alkaline media; DT50 (20C) (calc.) 8 d (pH 1), 12 d (pH 13). In natural sunlight, DT50 >40 d
pKa: 2.0, v. weak base.
F.p.: >150C.
APPLICATIONSBiochemistry: Photosynthetic electron transport inhibitor at the photosystem II receptor site. Maize tolerance of triazines is attributed to conjugation with glutathione.Mode of action: Herbicide, absorbed mainly by the roots.Uses: Broad-spectrum pre- or post-emergence weed control in maize, sorghum, vines, fruit trees, citrus, coffee, oil palm, cocoa, olives, potatoes, peas, beans, sugar cane, rubber, and in forestry in tree nurseries and new plantings. It remains largely in the topsoil, controlling a wide range of weeds, at rates of 0.6-3 kg a.i./ha; high rates are only recommended as band applications.Phytotoxicity: Phytotoxic to many annual plants and to aquatic plants.Formulation types: SC; WG.
MAMMALIAN TOXICOLOGYOral: Acute oral LD50 for rats 1590->2000 mg/kg.Skin and eye: Acute percutaneous LD50 for rats >2000 mg/kg. No skin or eye irritation. Not a skin sensitiser.Inhalation: LC50 (4 h) for rats >5.3 mg/l air.NOEL: (1 y) for dogs 0.4 mg/kg b.w. daily; (lifetime) for rats 0.22 mg/kg b.w. daily; (2 y) for mice 15.4 mg/kg b.w. daily.ADI: 0.0022 mg/kg.Toxicity class: WHO (a.i.) III (Table 5); EPA (formulation) IIIEC hazard: (R22)
ECOTOXICOLOGYBirds: Acute oral LD50 for ducks and quail >1000 mg/kg. Dietary LC50 (8 d) for ducks and quail >5620 ppm.Fish: LC50 (96 h) for rainbow trout 3.8-4.6, bluegill sunfish 7.5, carp and catfish 7.0 mg/l.Daphnia: LC50 (48 h) 21-50.9 mg/l.Algae: EC50 (72 h) for Scenedesmus subspicatus 0.016-0.024 mg/l.Bees: LD50 (oral and contact) >100 ug/bee.Worms: LC50 (7 d) for earthworms >200 mg/kg soil.Other beneficial spp.: No effects on bacterial respiration and nitrification in range 10.9-109 mg/kg soil.
ENVIRONMENTAL FATEAnimals: In mammals, following oral administration, 72-84% is eliminated in the urine and faeces within 24 h, and almost all within 48 h. A de-ethyl metabolite forms rapidly, followed by conjugates of products formed by oxidation of one methyl group of the tert-butyl moiety. All are rapidly excreted.Plants: Triazine-tolerant plants (e.g. maize) rapidly de-chlorinate terbuthylazine to hydroxy-terbuthylazine. Various amounts of de-ethylated and hydroxy de-ethylated metabolites are produced, depending on the plant species.Soil/Environment: Adsorption on soils is strong: Koc 162-278, Kd 2.2-25 are typical values for light agricultural soils. Terbuthylazine is only slightly mobile. Microbial degradation proceeds mainly by de-ethylation and hydroxylation, with eventual ring cleavage. DT50 30-60 d in biologically active soil.
Terbuthylazine (Tech.97 % pure) herbicide